As we get older, the fear of marked cognitive decline for many is greater even than the fear of dying. At a recent friends dinner many of us recounted our experiences with a parent who suffered cognitive decline. My wife and I share similar experiences as both of our mothers suffered disabling strokes in their 80s and 90s and both of our fathers had progressive and severe cognitive declines that were very painful to witness.
How can we better understand what dementia is and can we prevent it or slow its progression?
Dementia isn’t a specific disease but rather a term used to describe the development of a group of symptoms with impaired ability to think, remember, and problem-solve to make everyday decisions. It is caused by damage to the brain’s nerve cells and their surrounding connections and can have multiple causes.

Alzheimer’s disease
The most common form of dementia is Alzheimer’s Disease named after Dr. Alois Alzheimer, a German psychiatrist who in 1906 described the abnormal brain findings in a 50-year-old woman with presenile dementia; that is, dementia occurring at a much earlier age than expected. In 2020 it was estimated that this progressive degenerative condition of unknown cause affected 5.8 million Americans over age 65 and 55 million people globally. It is expected to rise significantly as more people will live longer over the next few decades.
As Alzheimer’s dementia develops, the brain typically shows progressive clumps of a beta-amyloid protein and fibrous tangles made up of a Tau protein. These tangles interfere with the communication points (synapses) between cells and can lead to cell death and loss of brain volume.
While the progression of cognitive decline is usually relatively slow, Lewy body dementia, characterized by the deposition of other proteins, is a form that progresses more rapidly and has an overlap with Parkinson’s disease.
Vascular dementia
The second most common form is vascular dementia, which usually refers to people who have had a loss of brain function due to a stroke. Mixed dementia refers to people with Alzheimer’s who may progress faster if they have abnormal brain blood flow due to atherosclerosis, which is the hardening of the arteries to the brain.
Other forms of dementia may include brain cell damage due to repetitive brain trauma, nutritional deficiency, immune disorders, or hydrocephalus, which is a build-up of pressure causing tissue shrinkage inside the brain.
What causes Alzheimer’s?
It is hypothesized that accumulation of the beta-amyloid protein in the brain may arise from various triggers and then leads to the development of tangles of Tau protein that affect communication between brain cells causing loss of function. While genetics plays a role in certain family clusters of early-onset dementia, its role in Alzheimer’s isn’t as clear.
It appears that multiple genetic abnormalities that affect inflammation, protein build-up, and ageing may each contribute. Advances in gene sequencing and the creation of large genetic databases of Alzheimer’s patients are providing insights into calculating the risk of developing the disease as well as helping to formulate targeted strategies to treat it before major irreversible damage occurs.
How is Alzheimer’s diagnosed?
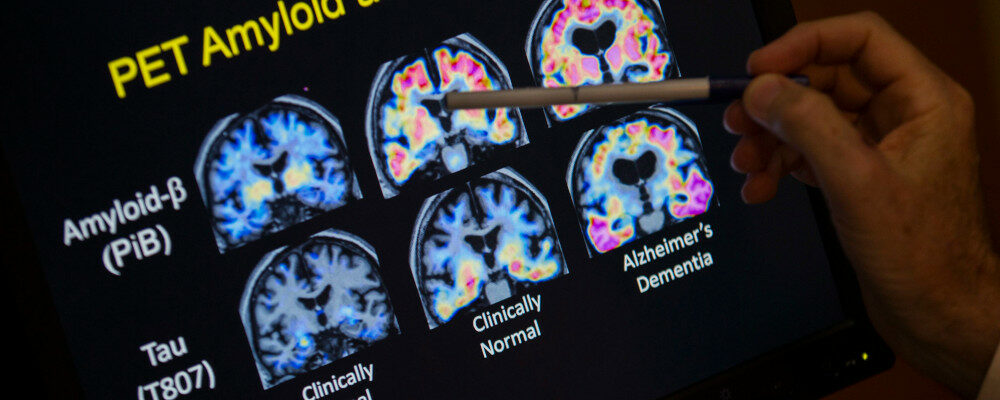
The diagnosis is typically made by demonstrating decreased performance in cognitive testing that evaluates memory and the ability to perform routine tasks. MRI scans may detect atrophy or shrinkage of the brain which may be non-specific and not just due to cognitive decline. PET scans can determine whether there is decreased metabolic function in different regions of the brain, and a specialized PET scan using targeted tracers can measure the build-up of abnormal amyloid or Tau deposits in the brain. A challenge is that not all people with amyloid protein on scanning have evidence of cognitive decline. While such scans can detect abnormal protein deposition they don’t always predict disease severity.
Preventing dementia
Modifiable risk factors for dementia patients are easier to define for vascular dementia. Stroke risks can be reduced by eating a healthy diet, regular exercise, avoidance of excessive alcohol intake, control of diabetes and hypertension, lowering cholesterol, and avoidance of smoking.
There are conflicting studies on the benefits of a healthy Mediterranean-type diet in reducing the risk of Alzheimer’s disease. While such a diet is healthy in many ways in reducing the risks of atherosclerosis, food-based studies are difficult to do given the lack of vigorous control groups and high-quality data on compliance as well as the inability to control for confounding variables. Eating a healthier diet is clearly good for you but at best only has a small role to play in maintaining brain health beyond stroke prevention.
Blood tests
Newer blood tests may provide insight into the risk of developing Alzheimer’s. Measurement of a protein, p-Tau217, in the blood may help identify those at risk of Alzheimer’s and track progression; however, their value is not yet fully defined. This newer blood test measuring Tau protein may soon be marketed as a direct-to-consumer option. It is more accurate than what is offered by the 23 and Me personal genome test which evaluates a different and less specific genetic protein abnormality. There are risks and benefits to having this potential knowledge about the lifetime risk of dementia when treatment options are limited and rates of gene expression and progression are unclear.
Conventional drug treatment
Conventional drugs targeting the treatment of Alzheimer’s have limited benefits, significant side effects, and work best in the early stages of the disease, despite the many TV commercials anecdotally touting their use. Because there are decreased levels of a brain chemical messenger, acetylcholine, drugs that are cholinesterase inhibitors such as Aricept, Razadyne, and Exelon are commonly prescribed. They work best in early disease and can cause nausea, vomiting, diarrhea, and cardiac arrhythmias. Their benefits are limited.
A second class of drugs such as memantine regulates the activity of glutamate, a brain messenger chemical, and may be useful in moderate Alzheimer’s if the significant side effects can be tolerated.
Exciting new drugs
Traditional drugs don’t deal with the underlying causes of dementia. Newer approaches look to try and remove amyloid and Tau proteins that have accumulated in the brain on the assumption that they are the cause of the disease.
The FDA in a controversial decision recently approved the use of Aducanumab (Aduhelm), the first new drug approved in 18 years for people with mild Alzheimer’s. It is a drug that is a human antibody administered intravenously that sticks to the amyloid protein, followed by your body triggering an immune reaction to remove it. The controversy and decision by some insurers to not pay for it relate to cost/benefit and side effects.
The drug is not benign and can cause brain swelling and micro bleeding. It is highly expensive, costing tens of thousands of dollars a year, thus limiting potential use. More worrisome is that the improvement in cognition wasn’t nearly as great as the removal of abnormal proteins. More studies are underway to see if earlier use before permanent damage may be more beneficial. Levanamab is a similar drug undergoing extensive testing.
Dementia remains a highly challenging disorder that has tremendous societal and personal costs to patients, families, and caregivers. While newer tests have improved diagnostic accuracy, they remain somewhat imperfect predictors of preclinical disease and progression. After many frustrating years of little progress in treatment options, a combination of genetics, advanced imaging, and new therapeutic targets finally show some light at the end of the tunnel. New applications are being studied that help to clarify the optimal timing of drug initiation and effectiveness in different ethnic populations.
In the meantime building a cognitive reserve that softens the decline and keeping healthy and fit to enjoy life as best we can, remains crucial. We need to remember that those affected need our care, love, and emotional support despite the painful challenges. Rather than living in fear of decline, we need to live the life we have to its fullest.